Innovation bottlenecks
Purified proteins are critical for driving innovation across sectors—from pushing the boundaries of precision medicine and fortifying crops against pests and diseases to optimizing the production of eco-friendly biofuels.
Advancements in artificial intelligence now allow us to engineer and predict protein sequences, significantly expanding the scope of potential applications. However, the primary challenge remains the production and validation of these proteins; for instance, databases like UniProt contain hundreds of millions of predicted protein sequences, yet less than 1% of these have been experimentally validated at the protein level, highlighting a critical bottleneck in translating theoretical models into practical solutions.
Existing cell-based protein synthesis methods are widely employed for routine protein production, but they face several limitations that make them unsuitable for bridging the gap in protein synthesis[1]:
Scalability challenges
- Scaling up cell-based systems for high-throughput protein production can be prohibitively expensive and technically complex, limiting their use in large-scale applications.
Limited protein types
- These methods often struggle with the synthesis of proteins that are toxic to cells, highly complex, or contain multiple disulfide bonds, such as many membrane proteins and glycoproteins. Post-translational modifications in cell-based systems are dependent on the host cell's biology, which can restrict the types of modifications that can be introduced and their consistency.
Scalability and cost
- Cell-based synthesis generally requires lengthy cell culture periods, which slows down the overall protein production process, hindering rapid testing and development. Maintaining cell lines and the necessary biocontainment measures can be costly, particularly when producing proteins that require specialized or pathogenic hosts.
While the production of oligonucleotides has become democratized, leading to widespread and cost-effective synthesis, protein synthesis has not reached similar levels of accessibility. As a result, the vast majority of predicted protein sequences have yet to be validated through purification, crystallography, or other analytical methods.
Cell-free protein synthesis: catalyzing innovation
In the early 1960s, researchers began dissecting the protein synthesis mechanisms in bacteria such as E. coli[2]. This pivotal research led to the creation of a technique known as cell-free protein synthesis (CFPS), which can produce any protein in the laboratory without the use of living cells.
At its core, CFPS involves producing proteins independent of the complexities and viability of living cells[3]. It utilizes macromolecular contents from lysed cells to perform the necessary chemical reactions (Figure 1).
The key components include:
- Cell lysates: These contain essential macromolecular elements needed for protein synthesis, from ribosomes to polymerases, extracted from various prokaryotic and eukaryotic cells[4,5]. The choice of system depends on the class and specific features of the target protein.
- DNA templates: This contains the gene that encodes the protein and other sequences that enhance transcription and translation. Both plasmids, which are more stable and yield more protein, and cheaper, faster-produced linearized DNA can be used, with methods available to protect DNA from degradation[6,7].
- Buffer solution: Optimal buffer solutions contain cofactors, ions, and a consistent ATP source to support and sustain gene expression, boosting protein yields.
- Amino acids: Amino acids are the building blocks of proteins. In addition to the 20 canonical amino acids, CFPS systems can also incorporate non-canonical amino acids, allowing for the production of proteins with specific structural conformations.
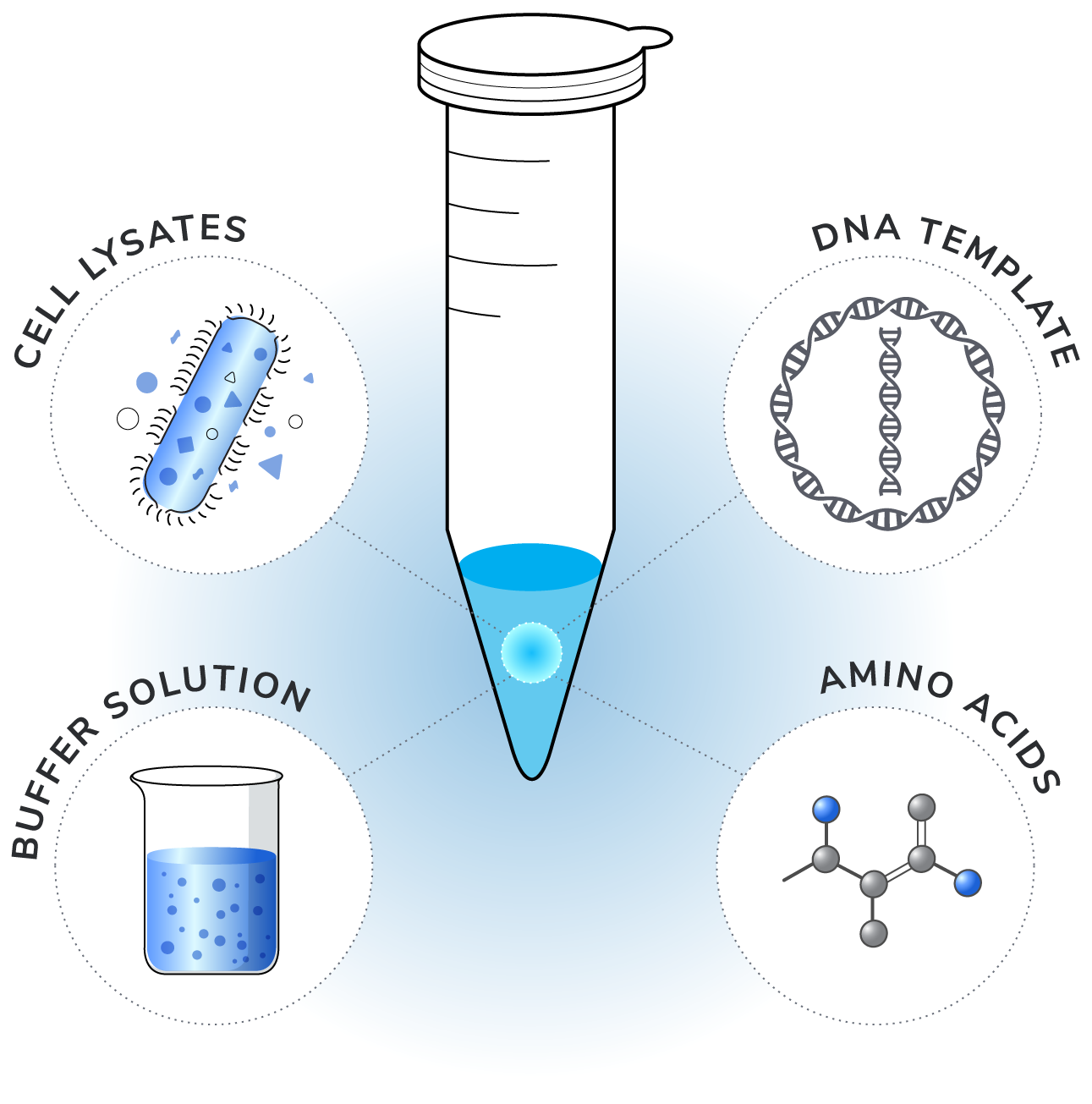
Optimizing the yield of a desired protein in cell-free protein synthesis requires meticulous tuning of several parameters, including the composition of cell lysates, the choice of DNA templates, the formulation of buffer solutions, and the types of amino acids used. Additionally, factors such as reaction temperature, incubation times, and the concentrations of enzymes and substrates must also be carefully regulated to enhance the efficiency of the necessary chemical reactions.
Advantages of CFPS
While traditional cell-based synthesis can be resource-intensive and limited, CFPS offers several significant advantages (Figure 2):
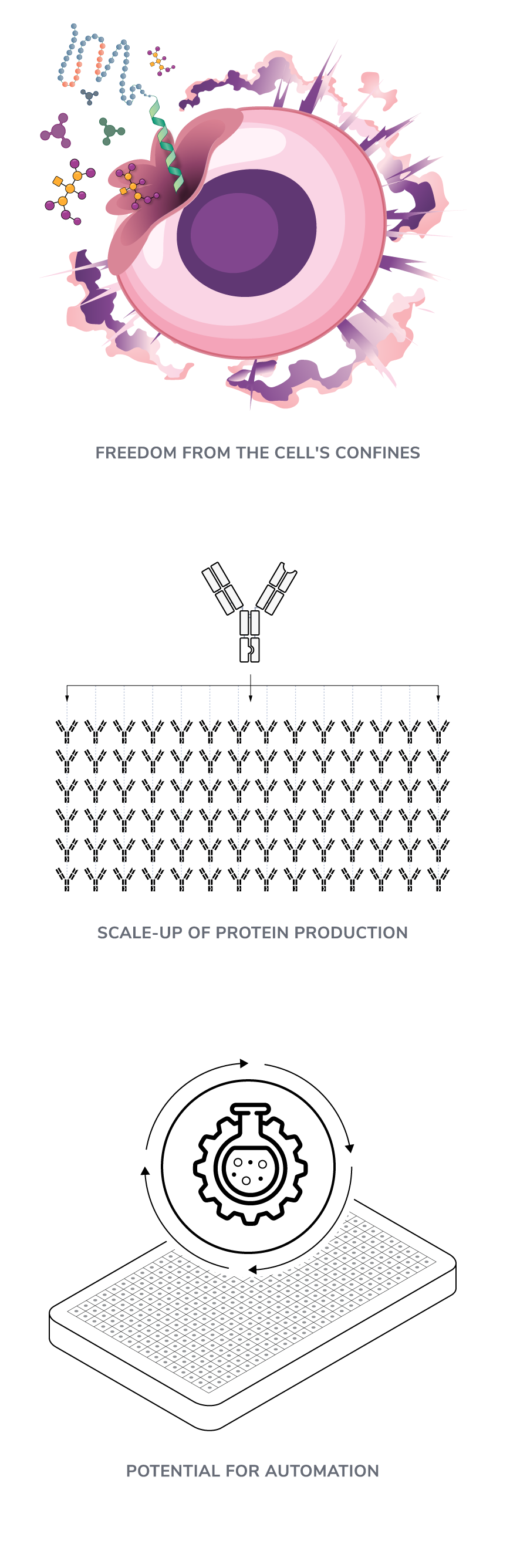
- Freedom from cellular limitations:
In cell-based expression, protein yield is heavily influenced by the overall health of the cell. Factors such as metabolic strain and cell-to-cell communication can significantly impact growth and productivity, creating a precarious balance that can affect output[9].
The cellular environment is also inherently complex, requiring sophisticated engineering and purification strategies to produce proteins. This includes the need to navigate the intricacies of the cell wall, as well as the requirement for precise genetic modifications and regulatory elements to synthesize complex proteins efficiently.
Cell-free manufacturing closely resembles chemical reactions that, unlike biological processes, are easier to understand and control[3]. - Scalability and cost efficiency:
The inherent heterogeneity of cells can lead to inconsistent protein yields and quality, which complicates efforts to scale up production and streamline process optimization. CFPS substantially reduces synthesis time and allows for concurrent production of multiple proteins, making the process more scalable and efficient. - Potential for automation: Cell-free synthesis operates cellular machinery within controlled liquid environments and are therefore compatible with automation and high-throughput systems.
Next-generation therapeutics, powered by CFPS
Drug discovery faces formidable challenges, including the high costs and slow pace of traditional development methods, compounded by the complexity of biological systems. Many therapeutic proteins and their targets remain poorly characterized, hindering the development of effective treatments.
By facilitating the rapid synthesis and characterization of proteins—including those that are difficult to produce in cell-based systems—CFPS accelerates the exploration and validation of potential drug targets. This method allows for precise control over the production environment, enabling the incorporation of non-standard amino acids and post-translational modifications that enhance drug efficacy.
As the pharmaceutical industry increasingly focuses on biologics and personalized medicine, CFPS offers a scalable and flexible platform, reducing development timelines and promoting innovative therapeutic solutions. Examples of these include:
Antibodies
- E. coli-based CFPS systems are capable of producing IgG antibodies that include noncanonical amino acids, creating potent antibody-drug conjugates[10]. These processes can be scaled up to milligram quantities in bioreactors compatible with standard industry practices[11].
For example, Sutro Biopharma utilized CFPS to address the shortcomings of traditional cell-based methods, enabling the rapid and scalable production of complex antibody-drug conjugates such as Luveltamab tazevibulin (Luvelta). By incorporating non-natural amino acids and precise protein modifications, they significantly enhanced the therapeutic efficacy of the resulting biopharmaceuticals.
Vaccines
- The COVID-19 pandemic underscored the urgent need for rapid and reliable vaccine production. CFPS has been instrumental in developing various vaccines efficiently and cost-effectively. Notably, it has been used to produce the major outer membrane protein of Chlamydia spp., a major vaccine antigen, using an E. coli-based CFPS[12].
Antimicrobial compounds
- In the ongoing battle against antimicrobial resistance, CFPS plays a crucial role. E. coli-based CFPS systems have been used to produce antimicrobial colicins and peptides that target antimicrobial-resistant pathogens[13].
Futureproofing CFPS
The success of CFPS platforms hinge on a range of critical parameters, each influencing the overall efficiency and output of the process[14]. Key among these are gene design, where synthetic DNA and regulatory elements like the internal ribosome entry site (IRES) from viruses boost protein yields by facilitating cap-independent translation. Reaction conditions also play a pivotal role; variables such as temperature, plasmid concentration, and ion levels must be optimized to enhance protein synthesis rates and quality.
As CFPS transitions from laboratory experiments to robust production platforms, several challenges and future directions become apparent:
- Quality and stability:
Advancements are needed in understanding and improving the components of CFPS lysates to enhance stability and quality. - Efficiency of synthesis:
Key factors such as cell-lysate quality, optimized reaction conditions, and template designs significantly influence protein yields and functionality. - Cost and scalability:
Reductions in the costs of essential components like energy systems, cofactors, and nucleotides are crucial. Additionally, the scalability of CF systems must be addressed to meet industrial demands.
The next phase of CFPS will likely see major strides in streamlining the synthesis of complex proteins, particularly through improvements in eukaryotic systems and the effective coupling of translation and translocation processes[15].
Moreover, integrated platforms that leverage AI can predict optimal conditions for CFPS, manage big data from high-throughput experiments, and automate the labor-intensive parts of the synthesis process, leading to more efficient and scalable protein production.
These platforms will not only refine how we approach drug discovery but also significantly lower production costs by optimizing the use of materials and energy sources. As CFPS technology matures, it promises to revolutionize the production of biologics and facilitate the rapid deployment of therapeutic proteins, offering a robust solution to meet the growing demands of modern medicine and biotechnology.
Unlock innovation with the Tierra Protein Platform
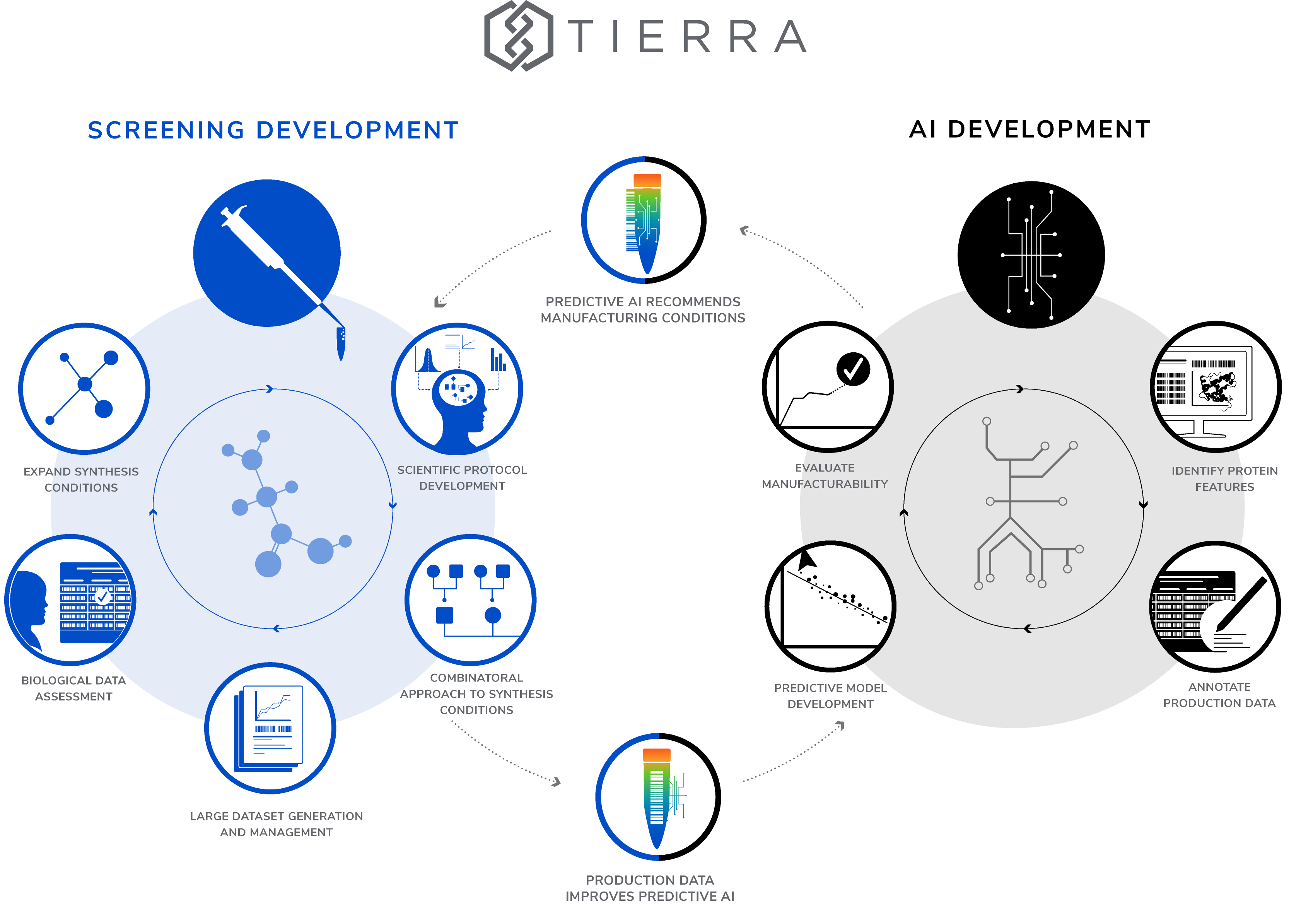
The Tierra Protein Platform revolutionizes scientific discovery by offering a fully integrated, AI-enhanced system that accelerates protein synthesis. Tierra’s proprietary technology provides seamless access to a broad spectrum of proteins, employs cell-free expression systems, and streamlines the identification of potential hits through automation, high-throughput techniques, and advanced computational analysis.
Comprehensive resources
- Tierra provides access to an extensive array of CFPS reagents, including diverse microbial cultures, specialized cell lines, and an array of lysate additives and solubility tags. Optimal components are selected to support robust protein synthesis while minimizing degradation, ensuring high-quality outcomes for even the most challenging protein targets.
Advanced high-throughput capabilities
- Utilizing high-throughput automation and predictive modeling, Tierra dramatically streamlines the protein synthesis process. The platform can test hundreds of reaction conditions simultaneously, optimizing the protein synthesis parameters swiftly to maximize yield and purity. This capability significantly reduces the time and effort required to meet specific research and development demands.
Sophisticated data analysis and predictive modeling
- By categorizing proteins based on sequence features and analyzing trends on protein yields, purity, and solubility, Tierra develops predictive models that correlate specific conditions with protein characteristics. This ever-expanding database not only enhances the efficiency of current production but also supports the scalable production of novel proteins.
Integrated platform and rapid turnaround
- Tierra has successfully integrated these elements into a unified platform that optimizes protein synthesis across various scales. Leveraging machine learning-informed AI and extensive automation, the platform offers rapid access to a diverse range of custom, purified proteins. Customers can expect delivery of microgram to milligram quantities of proteins within as little as three weeks, streamlining the pathway from protein design to application.
Unlock the full potential of your research—input your protein sequences into our easy-to-use ordering platform or contact us to discover how our tailored protein production solutions can accelerate your projects.