Antibody-drug conjugates (ADCs) are ‘biological missiles’, combining the precision of monoclonal antibodies (mAbs) with the potent cell-killing ability of cytotoxic drugs to treat cancer. Since the FDA approval of the first ADC, gemtuzumab ozogamicin (Mylotarg), in 2000, the field has seen remarkable growth with 14 approved ADCs and over 100 candidates in clinical trials[1].
These advancements have been driven by the limitations of conventional small-molecule chemotherapies. Traditional chemotherapy indiscriminately attacks rapidly dividing cells that contributes to debilitating side effects[2]. In contrast, ADCs like For example, brentuximab vedotin (Adcetris) targets CD30-positive cancer cells, delivering its cytotoxic payload directly into the tumor cells through a targeted mechanism, significantly reducing the likelihood of side effects., unlike traditional chemotherapy, which indiscriminately attacks rapidly dividing cells, thereby significantly reducing the likelihood of side effects.
Nonetheless, developing ADCs from discovery to patient use involves numerous challenges. Cell-free protein synthesis (CFPS) platforms can streamline the production of ADCs by simplifying complex manufacturing processes, enhancing the stability of the products, and enabling precise incorporation of targeting mechanisms.
Understanding ADCs
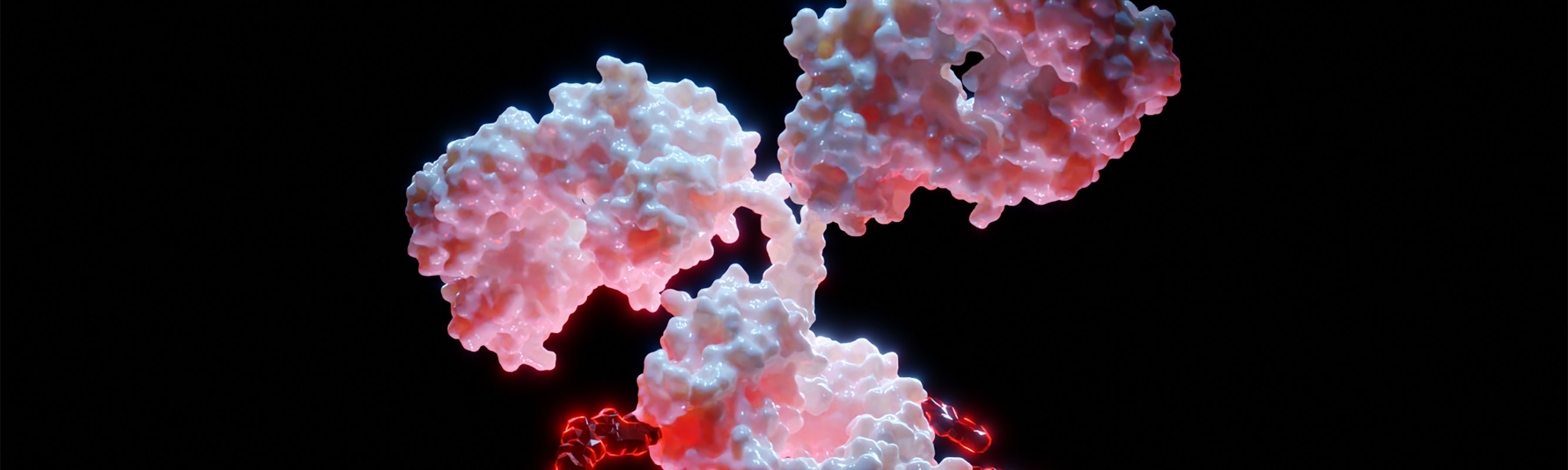
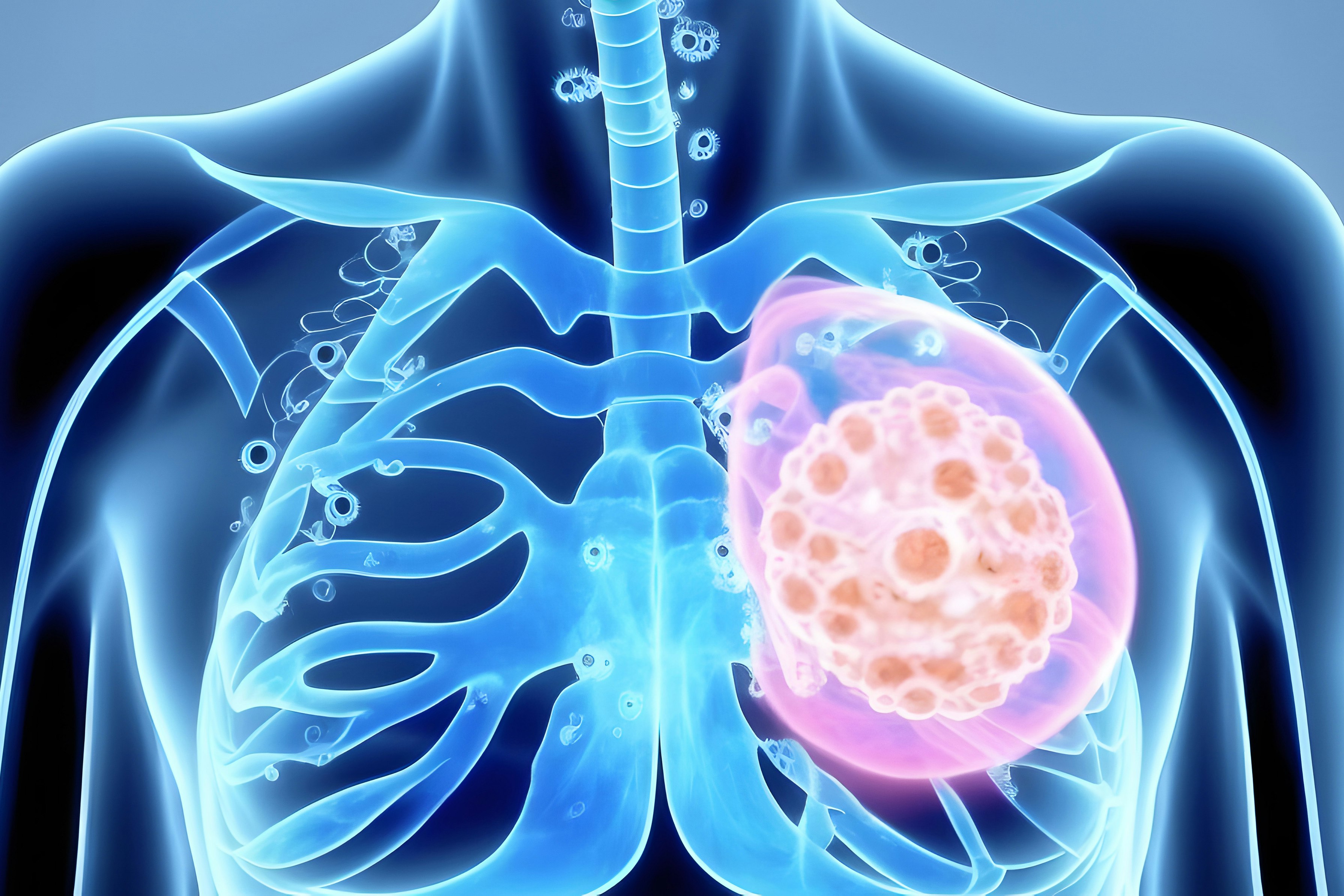
ADCs consist of a mAb linked to a cytotoxic drug via a chemical linker, forming a targeted therapy that delivers potent drugs directly to cancer cells (Figure 1)[3]. The mechanism of action involves the ADC binding to a specific antigen on the cancer cell surface, internalization of the ADC-antigen complex, and subsequent release of the cytotoxic drug inside the cell, leading to cell death (Figure 2)[4-7].
The clinical effectiveness of ADCs depends on several factors: the specificity and affinity of the mAb for the target antigen, the stability and release characteristics of the linker, and the potency of the cytotoxic payload. Optimizing these components is crucial for maximizing therapeutic efficacy while minimizing off-target effects.

Figure 1.

Figure 2.
Technical challenges in ADC discovery
Optimizing specificity and affinity
- The discovery and development of a novel ADC involves meticulously fine-tuning various parameters to ensure optimal therapeutic activity. Specificity is crucial; mAbs must selectively bind to antigens unique to tumor cells, which requires comprehensive profiling of cell surface proteins to identify overexpressed proteins in cancerous tissues[8].
Antigen display
- Equally important is the display of these target antigens on tumor cell surfaces. Antigens must be homogeneously expressed and adequately distributed to maximize mAb binding[9]. Internalization kinetics presents another challenge. Although tight antibody binding can enhance internalization, some ADCs, like trastuzumab in Kadcyla, exhibit slow internalization despite high binding affinity, necessitating further study of the factors affecting these kinetics[10].
Efficacy of the payload
- Finally, the cytotoxic payload must be highly potent, typically at sub-nanomolar or picomolar levels. The payload must also have physicochemical properties such as high hydrophobicity to facilitate diffusion into adjacent tumor cells and exploit the bystander effect for enhanced efficacy[11-12]. These parameters must be precisely calibrated to develop a novel ADC with optimal therapeutic potential.
Technical challenges in ADC development
The complexity of producing these bioconjugates also requires precise control over the conjugation process to ensure uniform drug-antibody ratios and stable linkages. Additionally, the inherent instability of some chemical linkers can lead to premature release of the cytotoxic drug, reducing therapeutic efficacy and increasing toxicity.
Manufacturing ADCs also involves handling highly potent cytotoxic compounds, necessitating stringent safety measures and specialized facilities. Furthermore, identifying suitable target antigens that are highly expressed on cancer cells but minimally on healthy cells remains a critical challenge, requiring extensive research and validation. These hurdles complicate the R&D process and demand innovative solutions to enhance ADC development and production.
The role of CFPS in ADC development
CFPS is an innovative approach that enables protein production without the need for living cells. Unlike traditional cell-based methods, CFPS utilizes cellular extracts containing the necessary machinery for transcription and translation, bypassing the complexities associated with cell growth and maintenance. This method allows for rapid protein production and is highly adaptable for incorporating non-canonical amino acids, offering significant advantages in the development of complex biopharmaceuticals such as ADCs.
Advantages of CFPS
Speed and Efficiency
- CFPS systems can rapidly produce proteins, significantly shortening the development timeline for ADCs.
- This method eliminates the time-consuming steps of cell culture and maintenance.
Flexibility in Incorporating Non-Canonical Amino Acids
- CFPS enables the incorporation of non-canonical amino acids into proteins, which is crucial for the development of ADCs with improved stability and functionality.
- These amino acids can facilitate more effective linker binding and cleavage, enhancing the overall efficacy of the ADC.
Enhanced Protein Folding and Assembly
- Initial efforts using CFPS involved identifying chaperone proteins that improve the folding and assembly of antibodies, such as trastuzumab.
- Proper folding is essential for the functionality of mAbs and their ability to bind to target antigens on cancer cells.
CFPS has already been employed to produce several ADCs. For instance, initial efforts to synthesize ADCs using CFPS focused on identifying chaperone proteins to enhance the folding and assembly of trastuzumab, ensuring its proper function and stability[13].
The potential and success of CFPS in producing ADCs is largely due to its capability to incorporate non-canonical amino acids into antibodies. This innovative approach enhances the functionality and stability of ADCs. Recently, E. coli cell lysates have been utilized to generate IgG molecules with these non-canonical amino acids, facilitating more efficient linker binding and cleavage[14]. This protocol not only ensures homogeneous ADC production but also streamlines the overall cell-free synthesis process, offering a significant improvement over conventional production methods.
Recent ADC developments
In 2024, the FDA approved several ADCs including fam-trastuzumab deruxtecan-nxki (Enhertu), which received accelerated approval for adult patients with unresectable or metastatic HER2-positive solid tumors who had exhausted other treatment options. Additionally, mirvetuximab soravtansine-gynx (Elahere) was approved for treating FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, specifically for patients who had undergone one to three prior systemic treatment regimens.
These approvals highlight the clinical validation and therapeutic potential of ADCs, offering new hope and more precise treatment options for patients with difficult-to-treat cancers. With advancements in enabling technologies such as CFPS, the development of next-generation ADCs will likely lead to more effective and accessible cancer therapies.
Work with us
Accelerate your scientific discovery with the Tierra Protein Platform. Our platform integrates automation, computation, and high-throughput screening enabling the efficient screening of antigen-binding domains to support the development of targeted and potent ADCs for cancer therapy. Visit our ordering platform or contact Tierra Biosciences today to learn how our platform and expert guidance can drive the success of your programs.